IFU Symbols Glossary
Symbol
Symbol Title
Symbol Description

Manufacturer
Indicates the medical device manufacturer
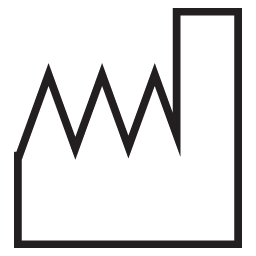
Date of Manufacture
Indicates the date when the medical device was manufactured.
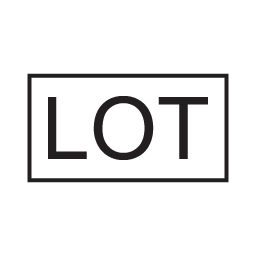
Lot Number
(Batch Code)
Indicates the manufacturer’s batch code so that the batch or lot can be identified.

Catalog Number
Indicates the manufacturer’s catalogue number so that the medical device can be identified.

Serial Number
Indicates the manufacturer’s serial number so that a specific medical device can be identified.

Device Not Sterile
Indicates a medical device that has not been subjected to a sterilization process.

Device Not Sterile
Indicates a medical device that has not been subjected to a sterilization process.

Sterilized with Irradiation
Indicates a medical device that has been sterilized using irradiation.

Do Not Re-Use
Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure.
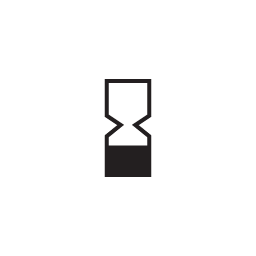
Use-by date
Indicates the date after which the medical device is not to be used.

Packaged Quantity
Indicates the amount of product included.

Material
Indicates material of the manufacturer.

Caution
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences.
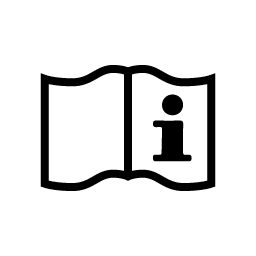
Electronic Instructions for Use
Indicates the need to the user to consult the Instructions for Use at: spinalelements.com/ifu/
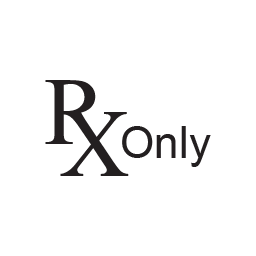
Prescription Only
CAUTION: U.S. Federal law restricts this device to sale by or on the order of a physician.
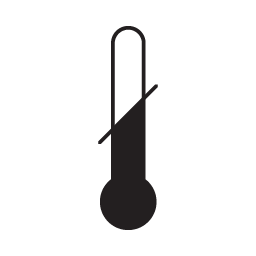
Temperature limit
Indicates the temperature limits to which the medical device can be safely exposed.

Authorized representative in the European Community / European Union
Indicates the authorized representative in the European Community / European Union.

CE marking – European Conformity
Indicates the device comply with the European requirements.

Do not resterilize
Indicates a medical device that is not to be resterilized
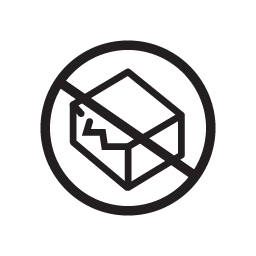
Do not use if package is damaged and consult instruction for use
Indicates that the medical device should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information.

Medical Device
Indicates the item is a medical device.
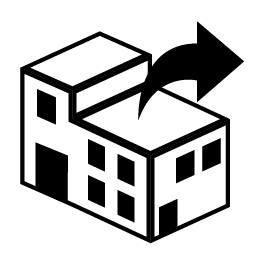
Distributor
Indicates the entity distributing the medical device into the locale.